Originally published in QEII Times.
Eliminating viruses with a nonthermal red light in the nose
Author: Jon Tattrie
The QEIIHealth Sciences Centre is testing out a new device that sanitizes your nose in minutes, with the goal of reducing the number of surgical site infections (SSIs).
“Surgical site infections are devastating. If you look at a surgical site infection in a total joint replacement, it’s a months-long recovery process,” says Dr. Andrew Glennie, a QEII orthopaedic surgeon and clinical champion for the Steriwave “test-and-try.”
SSIs happen when a germ gets into a patient undergoing surgery. Typically, they complete the operation and go home feeling well, but the infection can manifest up to 30 days later. Dr. Glennie says that often means they must return to the operating table to clean the infection, possibly remove/replace hardware, and face weeks of IV antibiotic treatment and a longer hospital stay.
Along with the hospital costs, the patient faces more time off work and other life obligations and challenges. “It’s extremely expensive,” Dr. Glennie says.
His department sees an infection rate between three and 10 per cent, depending on the complexity of the surgery and the underlying patient risk factors. This means that a surgeon will potentially treat 10 to 15 patients per year with invasive, multidisciplinary management of their infection. He says SSIs can come from a variety of different avenues including colonization within the operating room, but they can also come from the patient themself. Patients tend to clean their arms and hands inconsistently. At the QEII, patients have been offered swabs to decolonize their nose at home before surgery in the past, but compliance and competency are hard to track.
That’s where the Steriwave comes in. Natasha Breward, business development co-ordinator with the Nova Scotia Health Innovation Hub, says her team learns about such problems from doctors and then seeks solutions.
The Innovation Hub is a first-of-its-kind centre of excellence for health research and innovation in Atlantic Canada, working to deliver better solutions for patients and providers through the best available evidence and well-researched solutions.
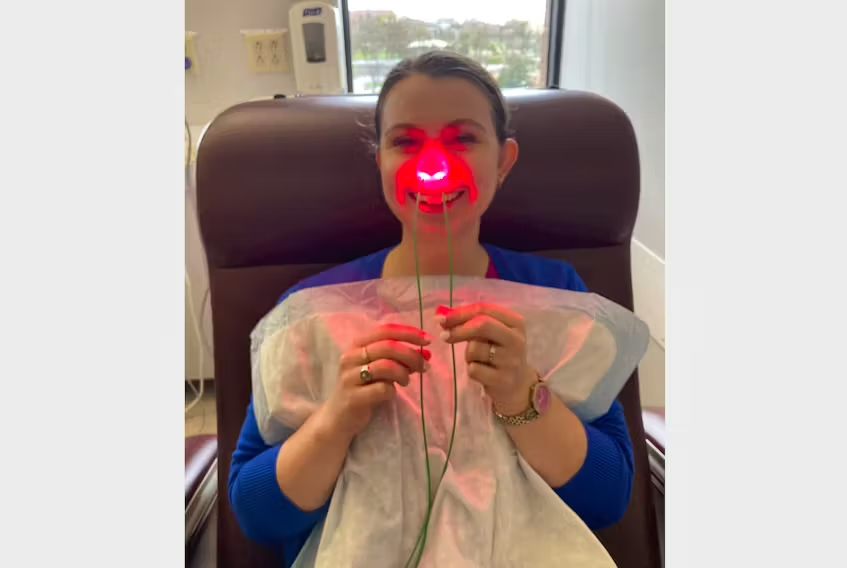
The Steriwave was developed by a Canadian biomedical company called Ondine Biomedical. Shortly before the operation, a nurse swabs a blue gel into the patient’s nose. They insert a small device, which uses a special light as antimicrobial technology to quickly destroy pathogens. Since it doesn’t rely on antibiotics, patients don’t have to worry about antibiotic resistance.
The QEII Foundation funded $50,000 to trial the device with 700 people who need orthopaedic or spine surgeries. The first patient tested it on Oct. 25 and the project will end in the fall of 2024.
Steriwave had been used in Vancouver and Toronto and shown good results, dramatically reducing SSI rates.
“This device uses nonthermal red light in the nose as well as a blue gel, and those two things combined together really eliminate the viruses, bacteria and fungi,” says Natasha.
“We hope it’s a no-brainer,” Dr. Glennie says. “Because the treatment of surgical-site infections is so morbid and expensive, the adoption of this technology will be a cost-savings venture.”
Natasha agrees. “Based off our evaluations of this year-long trial, we’ll be able to put forward a business case study to really highlight the positive impact it had in Nova Scotia Health.”
Every patient who goes through surgery could benefit from the extra step, she says. It takes five minutes but could save months of recovery. The nurse does it with them as they sit in the pre-operative area, meaning compliance is essentially 100 per cent, and it’s always done properly.
Tanya Rodgers, the health services manager who was key to bringing Steriwave to Halifax, says she hopes the results confirm initial findings.
“The treatment is provided in five minutes and the patient has long-lasting benefits. So, it is a no-brainer for us and the nurses are very excited to be involved.”
If the trial is a success, the process could be used with patients preparing for every surgical procedure at the QEII.